{{item.title}}
{{item.text}}

{{item.text}}
Authors:
Tory Smithe, Head, Digital Strategy - Healthcare & Life Sciences, Adobe
Jonas Sjoquist, Life Sciences Strategy Lead, Adobe
Tyler Jorgensen, Principal, Customer Transformation, PwC US
Tyler Mansfield, Principal, Customer Transformation, PwC US
The stakes have never been higher for pharmaceutical companies to be able to efficiently personalize omnichannel experiences, reach relevant patients faster, and provide healthcare professionals (HCPs) and patients with the information they need to enhance the treatment journey.
As access to HCPs becomes increasingly constrained and vying for their attention highly competitive, pharmaceutical companies are under significant pressure to provide content that is not only timely but also highly relevant and tailored to customers’ needs. Traditional methods of content creation in the industry can be highly manual and inefficient. They also fail to extract meaningful insights due to incomplete customer datasets and limited understanding of historical performance, which can lead to disjointed communication with target HCPs and patients.
Imagine a model where you could go from brief to deployment in less than 24 hours, with intelligent, automated content reviews driving compliance with pre-defined MLR standards from the start. Imagine market-ready content on Day One of launch that helps deliver a highly personalized experience for customers across every channel and touchpoint. With the right approach, this can be achievable. It’s all powered by a consolidated technology stack, streamlined workflows, and the latest AI models applied across the end-to-end (E2E) content lifecycle. This new way of working allows teams to focus on what matters most: deliver impact on a scale faster than ever before.
The present conditions are fertile ground for technology adoption. These trends are reshaping how pharma should adapt to meet evolving expectations:
Pharmaceutical companies operate in a highly technical, highly regulated environment, where safety and precision are integral to content management, and even minor inaccuracies can lead to compliance risks, regulatory scrutiny or even patient harm.
Technology solutions should accelerate and orchestrate workflows across the end-to-end lifecycle and maintain the depth, accuracy, and regulatory alignment required in a complex space. To achieve this, some unique additional capabilities are required:
1. Medical claim creation, content libraries and tactic assembly
2. Automated MLR readiness and review
3. Integrated regulatory submission
Adobe GenStudio is a first-of-its-kind offering: a single, fully consolidated platform that spans the end-to-end content lifecycle. It harnesses the power of GenAI to streamline content creation, allowing brands to produce diverse, on-brand marketing materials quickly and efficiently. Its AI-driven capabilities enable marketers to generate and remix content in various formats for personalized and high-performance campaigns across channels and tactics.
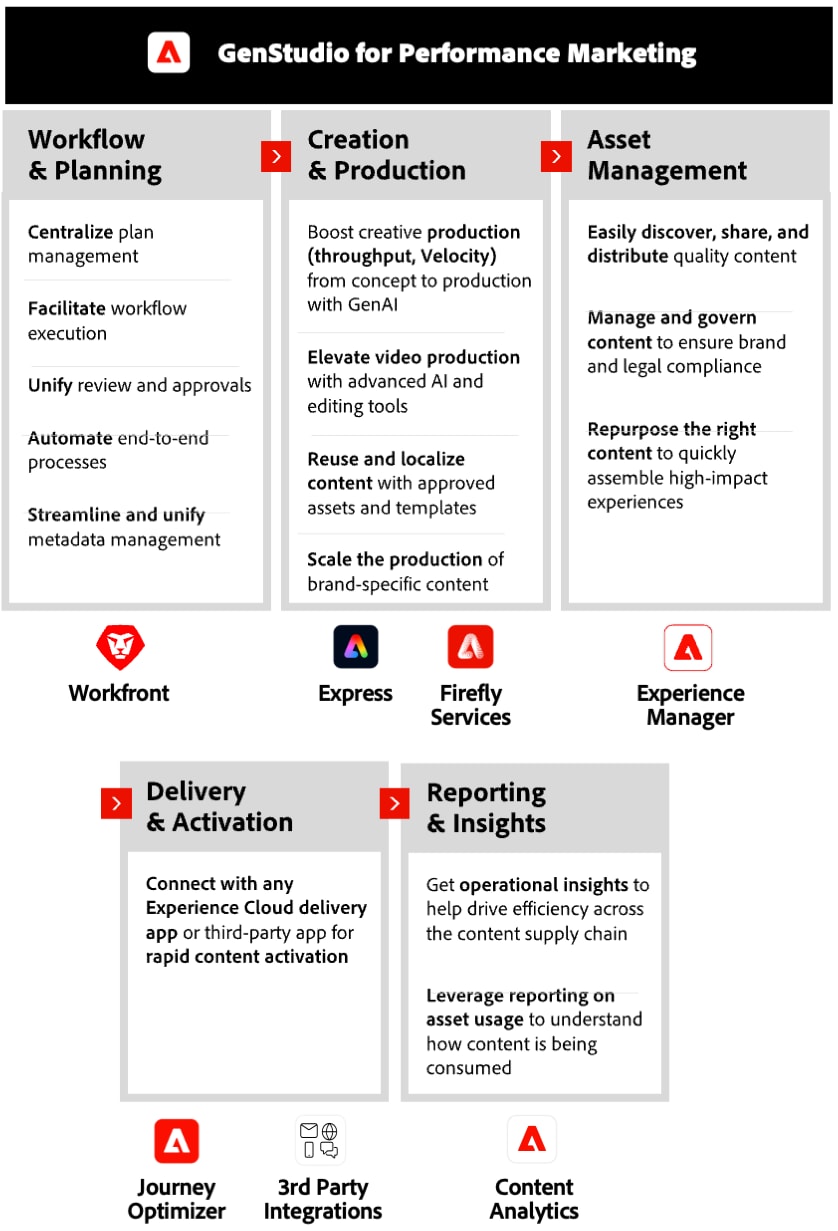
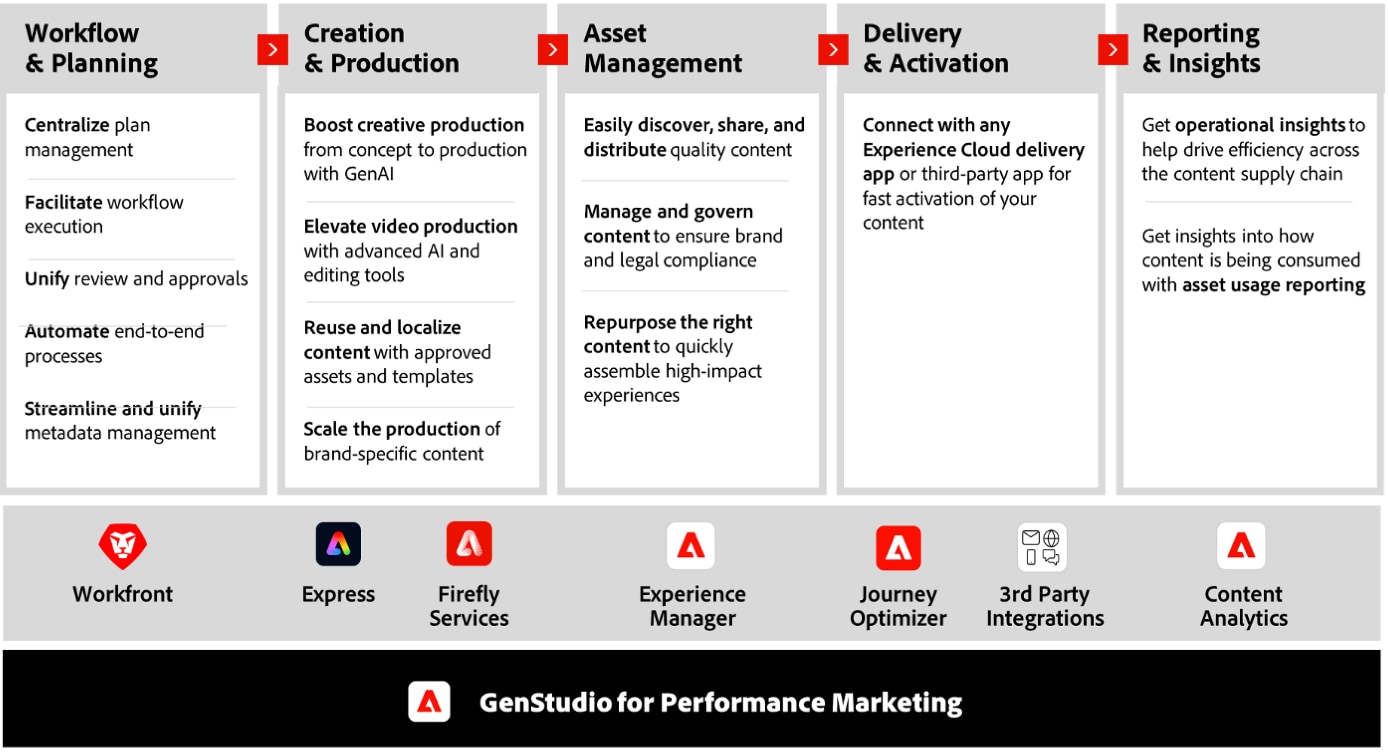
PwC leverages deep pharma industry experience to help streamline content management and offer tailored solutions, flexible enough to handle future challenges. Using large language models (LLMs), our accelerators generate relevant and compelling product claims and support "glocalization" or "local-to-local" content strategies. These tools help drive MLR readiness with an intelligent pre-screen, covering requirements for tactic assembly and integrating regulatory submissions into a seamless, end-to-end solution.
Pharmaceutical companies are at different stages in their content management journey—some building on existing systems, others undergoing vast transformation. Regardless of where they stand, the goal remains the same: to streamline workflows, enable modular content, and leverage GenAI for greater efficiency and speed. The path to a future-ready content model isn’t one-size-fits-all; it’s a strategic decision to gain a competitive edge through agility and innovation.
This transformation goes beyond technology—it’s a fundamental shift in how pharma organizations collaborate and operate. To realize the benefits, companies should embrace an agile, tech-empowered ecosystem that fosters collaboration across marketing, medical, legal, and regulatory teams, ultimately:
Now is the time to replace bottlenecks and inefficiencies with intelligence, precision, and speed. AI can handle manual reviews and content pre-screens, freeing marketers to focus on high-impact messaging and MLR teams to take on a role of strategic oversight.
Ultimately, the pharma industry’s ability to unlock the full potential of AI-powered end-to-end content management will depend not just on technology adoption, but on its willingness to rethink how teams collaborate, how decisions are made, and how innovation is embraced to step into the future.
{{item.text}}

{{item.text}}