{{item.title}}
{{item.text}}

{{item.text}}
The pharmaceutical industry has plenty to celebrate. In the last decade, major therapeutic advances, such as immunotherapy and cell and gene therapy, have given new hope to patients. COVID-19 vaccines were developed in record time to help save the world during a historic pandemic.
But during that same period of groundbreaking innovation, pharmaceutical companies failed to keep pace with the capital markets. In fact, returns from pharmaceutical companies lagged the S&P 500 by about one-third, and biotech fared even worse.
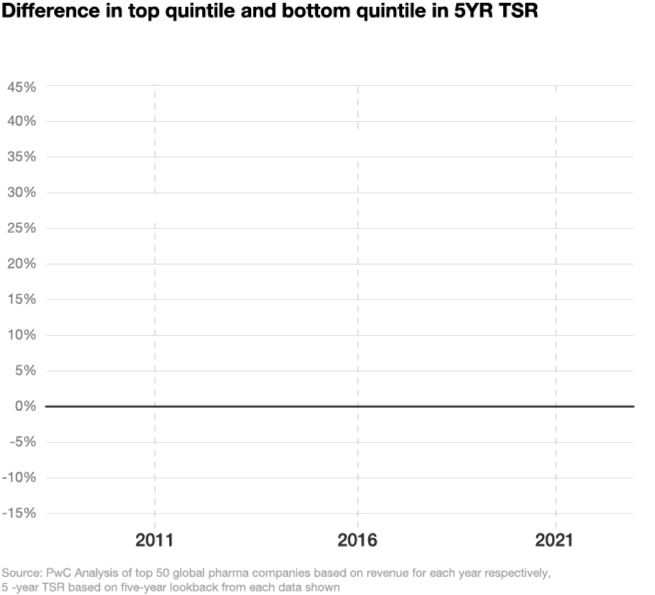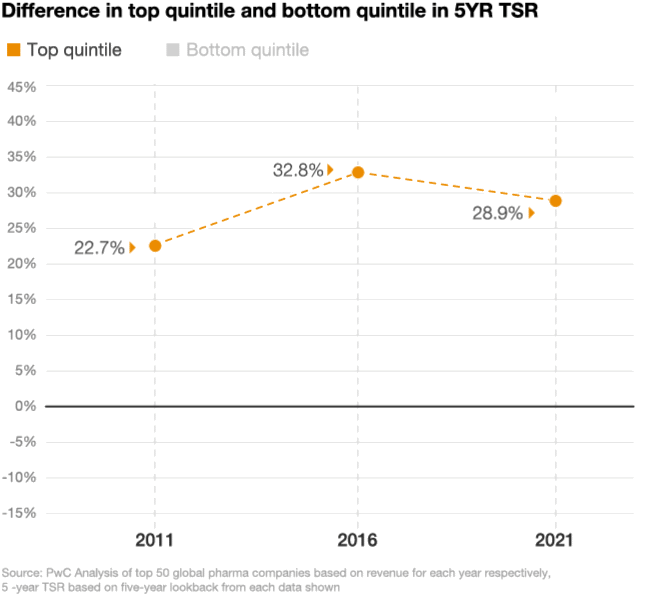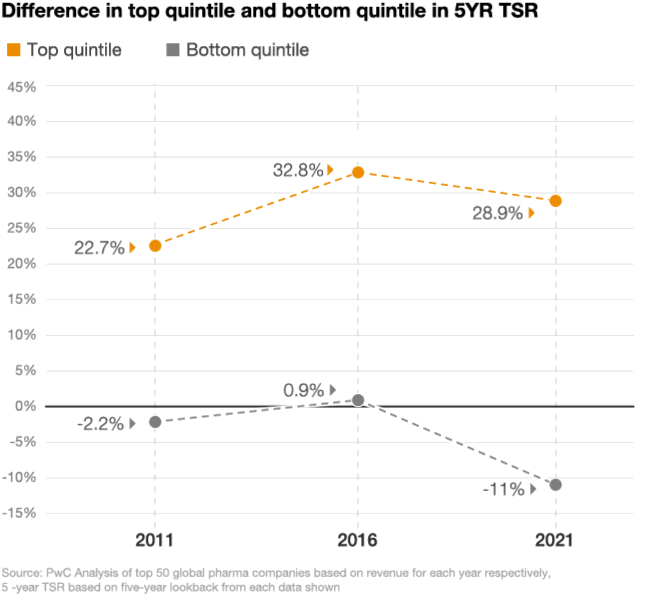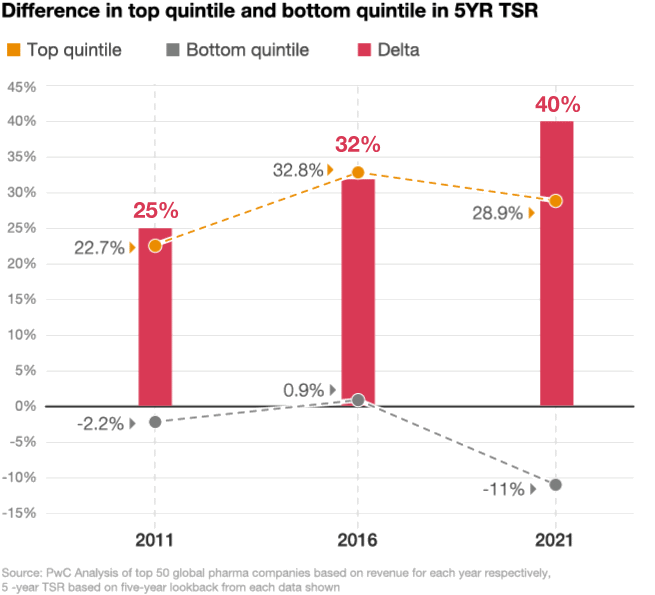
Looking at the stock performance of the top 50 pharmaceutical companies, the divide between the leaders and laggards has been widening. In 2021, the five-year total shareholder return (TSR) for drugmakers in the top quintile was up by 29%, compared with a decline of 11% in the bottom quintile, according to a PwC analysis (figure 2). As performance pressures mount, investors are taking a closer look at which pharma companies are positioned to win and allocating their investments accordingly.
Market headwinds are well documented. Drugmakers are grappling with extended drug development timelines, pricing scrutiny, high costs of regulation and litigation, and increasing competition in nearly every category. Another wave of patent expirations is just around the corner, meaning more therapeutic areas will have generic or biosimilar alternatives. Gross margins for some novel treatments, such as chimeric antigen receptor T-cell (CAR-T) therapy, are well below historical averages and the increasing personalization of medicine means manufacturers need to generate returns on smaller populations of patients. All of these forces need to be overcome in pursuit of higher shareholder returns.
First, as leaders set the path ahead, they should incorporate a sharper lens on shareholder value creation into everyday decision-making. Connecting product-market decisions (e.g., portfolio choices, launch investments, production expansions) to shareholder value creation across the enterprise is essential to help translate great science into great returns.
Similarly, another overarching imperative will be delivering on the promise of digital innovation. PwC research shows results to date have been disappointing. Looking ahead, the pharmaceutical industry can learn a lot from leading tech companies in areas like developing digital products, personalizing customer engagement, harnessing new types of data, deploying intelligent automation and working in a more agile fashion.
With a foundation set on those goals, pharma leaders can gain competitive advantage by focusing on five key actions:
Key industry capabilities (e.g., decentralized trials, machine learning at scale) will help drive the business forward in a differentiated manner. Companies that execute flawlessly on these capabilities will have a competitive advantage.
In the age of “The Great Resignation” and the ”war for talent,” having a differentiated culture to attract and retain the right staff will be critical. Employees want to find purpose and know that what they are doing can drive change in the world. For employees at pharmaceutical companies, work means saving lives. Doubling down on this mission, while building a unique culture that matches your strategic imperatives is critical. Communicating and executing on those values daily will help drive the most sustained loyalty from your personnel.
Given the aforementioned challenges in the industry, it will be critical to minimize any downside risk around cybersecurity, regulatory challenges and legal matters in order to preserve value.
Companies have made massive investments in enterprise resource planning (ERP) systems, artificial intelligence (AI), automation and cloud. Now is the time to capitalize on these investments, driving them to the front-end patient experience to improve customer satisfaction, while also improving the efficiency of operations.
The industry spends a lot of effort and focus on determining where best to drive the business strategically (e.g., geography, therapeutic categories, scientific modalities). There is no doubt that making the right strategic choices can provide a lot of headroom, however, we see returns accelerated by better execution of divestitures as well as in building out the best-in-class partnership ecosystem to drive leading science, technologies and talent to the business.
Assets, such as patents and products, have always been important to value growth in the pharmaceuticals sector. But assets come and go. Capabilities are enduring and can create a lasting competitive advantage. As pharmaceutical companies look to outperform in the future marketplace, six capabilities are expected to be critical to success:
Patient-centric trial design is a hot topic today and is expected to be a defining capability for the future. The COVID-19 pandemic drove the industry to rethink the traditional paradigm for clinical trials, accelerating the development and use of decentralized design. The ability to design, execute and oversee clinical trials that run in the home, near the home in retail pharmacies and health clinics and virtually is a must-have. Decentralized settings reduce the burdens on participants, such as the need to travel to a major academic medical center, and therefore increase the number of individuals willing to participate in a study. More convenient and less burdensome trials also increase the odds of retaining participants throughout a study. Furthermore, decentralized trials hold great promise for reaching underrepresented communities, resulting in more relevant research in which the study population looks like the disease population in the real world.
While the potential impact for patients (less burden, more access to trials) and for sponsors (faster trial enrollments, reduced dropout rates, more competitive trial design) is significant, creating a winning capability in decentralized trials means adopting significant changes. R&D leaders should evaluate their portfolios to assess which trials are best suited for a decentralized design, change processes related to trial enablement, update the vendor ecosystem to include suppliers that specialize in decentralized trials and upgrade the use of data and analytics to support the trial life cycle. All of this will need to occur while balancing the ability to execute and oversee traditional randomized clinical trial activities. Those who get it right will be living the industry’s “patient at the center” mantra, while increasing their own odds of success in the competition to recruit and retain patients.
Most pharmaceutical companies have explored opportunities to apply robotic process automation (RPA) and have developed use cases that leverage artificial intelligence and other emerging technologies. But many pharma leaders see scaling these advanced technology plays beyond a handful of pilots as a challenge. Tackling a few high-value use cases in drug discovery or customer engagement helps, but the real opportunity is to reimagine the pharmaceutical company of the future, powered by intelligent technologies across the enterprise. The result will be an organization that can handle more sophisticated decisions with better outcomes and shorter time frames.
Leaders in tackling the human-machine possibilities will start with a clear, top-down vision of what they are trying to achieve. This might include vaporizing millions of hours worth of routine work across the enterprise, increasing speed on cross-functional processes or improving effectiveness. Enterprise-wide change requires a clear vision and specific goals. It also requires upskilling the business to have a stronger sense of what’s possible with predictive analytics and intelligent automation. Individuals should be well-equipped to generate and engage on ideas, and should have incentives to think differently. These technologies are powered by large data sets, so fit-for-purpose data strategies that are linked to the digital products and services they support can better enable scaling.
The drumbeat on value will continue into the future, meaning drugmakers need to enhance their value proposition. While advances in traditional measures of product efficacy and safety are still critical, digital innovations create new possibilities for helping physicians and patients achieve improved outcomes. Tracking digital biomarkers, offering digital therapeutics and innovative clinical decision support tools and creating connected treatment ecosystems offer new opportunities to predict disease progression, engage patients more deeply in their treatment and support their path to success.
Discovering, developing and commercializing these next-generation solutions is a different type of capability. Borrowing lessons learned from the tech industry, leaders in the pharmaceutical sector will have a deep understanding of human-centered design, device/solution regulatory pathways and ecosystem partners that can accelerate the journey. They will redefine operating models to incorporate digital solutions as part of product development and commercialization, starting early in the process. Beyond the direct value generated by these solutions, another important element of the value equation is the information that can be generated by these digital products. Winners in this domain will have great solutions, but they will also be world-class in envisioning what data will be valuable and putting the capabilities in place to take full advantage of it.
Real-world evidence (RWE) is undergoing rapid evolution as its impact deepens in clinical development and expands across the product development life cycle. As pharmaceutical companies look to convert major investments in RWE into real returns for patients and shareholders, the trustworthiness and fit-for-purpose nature of the data sources, the approaches used to convert observations to evidence and the need to upgrade organizational decision-making are important elements in determining the capabilities of RWE in the future.
Real-world data (RWD) and the evidence derived from it (RWE) have become progressively more sophisticated as the patient journey has become ever more digital—from structured data in insurance claims 20 years ago, to the widespread adoption of electronic medical records (EMRs) over the last decade and now to sources of near-real-time data directly from the point of care or from patients’ homes and phones. But more data has not always translated into better evidence, a fact that’s acutely understood by regulators and payers. To ensure this proliferation of data is of high quality and reliable, leaders will be out in front to define standards of trustworthiness for RWD and RWE.
Going forward, pharmaceutical companies have an opportunity to expand their collection of RWD and create the analytical capabilities needed to turn these new sources of data into compelling evidence. Beyond data and analytics, changing the organization’s mindset about evidence is a challenge. In the past, product and portfolio decisions often have been weighted toward experimental data from clinical trials and advice from key opinion leaders. RWD provides an additional view, and leaders should ensure this new class of evidence is incorporated into their company’s decision-making processes.
The early adopters of RWE are starting to reap the benefits of their investment, as regulators and payers increasingly recognize its benefits and learn to deploy it effectively. The rest of the pack is looking to leapfrog with vendors and partners that have already learned what didn’t work. Landing as a disruptor, and not as one of the disrupted, means having a leading capability in RWE.
With pricing scrutiny expected to persist, winning and retaining customers to drive volume will be essential to growth. And customers today expect a more digital experience. According to Wunderman Thompson’s research, more than three-quarters (76%) of global consumers say their everyday lives depend on technology, and this figure is even higher for Generation Z and millennials, at 79% and 80%, respectively. Eighty-one percent of global consumers say they “switch on” to unwind, and over half say they are physically (55%) and mentally (56%) healthier, thanks to technology.
As these trends and others like them continue, pharma’s commercial leaders have an opportunity to harness digital technologies to design the differentiated customer experience of the future.
Imagine a deeply immersive virtual reality environment where physicians can experience content (not just access it), interact with peers, ask questions of pharma staff and activate a range of support services. In contrast with a two- to three-minute detail by a sales specialist, this is an environment where a physician could spend a much more meaningful period of time. Predictive analytics would customize the environment for this physician, and orchestrate the right follow-ups based on the experience. Similar environments would be accessible to patients.
The next generation of experiences is just around the corner, and commercial leaders should ensure they are ready. This means strengthening human-centered design skills, shaping partnerships with new types of vendors such as metaverse players, creating branding concepts that are intriguing and unconventional to resonate in a digital-first environment and using the power of predictive analytics to identify and activate customers most effectively.
The coronavirus pandemic upended the traditional engagement model. Companies were forced to adapt in response to the crisis. Going forward, innovating the customer experience will be a source of competitive advantage.
If the pharmaceuticals sector is to outperform in the capital markets, it needs to boost the outlook for future profits and ensure plans are in place to deliver. How resources are allocated across the company is a big driver of both growth and returns. Are costs, capital and people allocated to their highest and best use, as measured by alignment to the strategy and ability to create shareholder value? In most large, complex global enterprises there are organizational realities that work against optimal resource allocation, such as fuzzy priorities, prior-year-budget inertia, “squeaky wheels” and gut-instinct planning.
The ability to align resources with their most valuable use year after year is a strategic capability that can convey a lasting advantage. But it is not easy. Value-based planning requires an objective understanding of where and how value is created in the enterprise (e.g.,products, markets, customers) and how resources are consumed by each of the units. Operational drivers of work (for example, speaker events, customer visits and scientific publications) should be connected to their true cost and tested for alignment to both strategy and value (realizing some activities cannot or should not be measured strictly by return on investment). Productivity should be analyzed through new lenses. For example, you could borrow the working-to-non-working spend ratio used in consumer products businesses to spark ongoing questions about how best to reallocate resources from lower-value to higher-value activities.
Traditional budgeting is not getting the job done, as seen in the sector’s lagging shareholder returns. Value-based planning offers CEOs and their teams a better capability for managing resources and ensuring all of the great work underway within the company translates into stronger results in both the product and capital markets.
The six essential capabilities can only be delivered at scale with the power of the cloud. Companies should create the data, analytics and technology environments to power the modern pharma and life sciences enterprise and support new capabilities, such as leveraging cloud native services to extract key information from documents and cost-effectively managing large volumes of near-real-time data.
Cloud platforms are typically leveraged by pharmaceutical companies for either infrastructure (migration to cloud for compute power and storage) or for their innovative tools (services) such as natural language processing, speech-to-text conversion and machine learning.
Pharma companies are currently in various stages of migration to the cloud infrastructure, a transition that promises improved efficiencies, expanded service offerings and lower costs for operations. Moving legacy applications to the cloud could cut the cost, but in order to realize these savings, companies must make substantial investments in modernizing their IT infrastructure to take advantage of cloud-native architectures for their aging legacy systems.
A more rapid path to cloud benefits is acceleration of innovation through cloud-native services. For example, voice-to-text conversion services along with natural language processing services can detect safety events and product issues through call center operations, improving quality and reducing review effort and time. Machine learning can be used to automate product complaints and adverse events processing, reducing effort and duration. These examples are real and just a taste of the benefits to be had leveraging cloud-native services.
In a 2021 PwC survey of health industry leaders, 42% of respondents said that improving the experience for patients was the main goal behind investing in cloud technology. Among other patient-friendly offerings, cloud computing supports the planning of decentralized trials, identifying aspects of trial design that will or will not work at remote sites, helping to minimize burdens and boosting the likelihood of success.
In the next five years we expect all of the top 20 pharma companies will adopt a comprehensive cloud strategy—encompassing migration to cloud infrastructure and cloud-native services to drive innovation.
Over the past decade, PwC’s annual global culture survey has consistently shown that organizations with a distinct culture deliver higher growth and profitability than their industry peers. Culture can either accelerate or hinder the types of transformational changes that will define the drugmaker of the future. So there’s no getting around the role of culture in driving an uplift to pharmaceutical sector performance.
While every company will make its own choices on the behaviors to emphasize in their unique culture, two themes will be important in the cultural underpinnings of tomorrow’s pharma company:
“Next-generation transformation at scale means every part of the enterprise will need to innovate.”
The first is trust. Trust is a precious commodity, especially for a sector that asks individuals to participate in scientific experiments, share personal data and pay significant amounts of money for the promise of improved health. Organizational behaviors aligned to building trust with external stakeholders such as customers, partners and regulators are critical to living the industry’s purpose, and also to realizing the value that can be created in areas such as digital engagement, real-world evidence and decentralized trials. In particular, behaviors to build trust in underserved and underrepresented populations will be increasingly important to both improving societal outcomes and accessing new patients.
Ensuring a culture of trust internally is similarly important. About half of the employees who participated in PwC’s 2021 Global Culture Survey reported not feeling listened to, seen or included at work. CEOs’ responses to the same survey revealed a much rosier outlook (figure 4). This disconnect tells us that managers might pay lip service to inclusion, but that employees aren’t feeling real effects from all the talk.
In an industry that relies on highly skilled knowledge workers, trust is essential for attracting and retaining talent, as well as bringing employees along in the transformation journey. Employees should believe that:
The leaders of the future should nurture a deep sense of belonging and create a bond of trust with their people. Being bold and bringing back the feeling of pride to the industry will be critical to not only maintaining current employees, but becoming the employer of choice.
The second key cultural theme is innovation. Of course pharma is innovative, right? Concerns about taking risks and going outside of traditional comfort zones can put a damper on new ideas, especially in large-scale, established companies. Next-generation transformation at scale means every part of the enterprise will need to innovate.
Emphasizing behaviors related to reimagination, creative destruction, lifelong learning and new types of multidisciplinary teaming will help to counteract institutional forces that can squash innovation. No corporate center is powerful enough or all-knowing enough to drive change exclusively from the top. Along with significant business-led investments, innovation will come from digitally upskilled employees who are close to the work, committed to the mission and feel empowered to pursue new ideas.This type of citizen-led innovation has the potential to become a self-reinforcing system of innovation and value creation. Encouraging these behaviors is a must for drugmakers who expect to be on the cutting edge of both science and business.
A culture deeply rooted in trust and innovation will be an accelerator in the journey ahead. The leader’s work is to ensure these themes are infused into everyday organizational behaviors in a way that creates a lasting advantage for the company.
Transactions have always been the fabric of the pharma industry, and deal activity will accelerate as companies look to inorganic activities to achieve their growth plans. Transactions for products as well as capabilities are on the agenda for all CEOs. With the continued scrutiny of the US Federal Trade Commission (FTC) on larger deals in pharma, most companies will need to drive a higher number of transactions to get the same outcomes.
While every company has structures and funding to maximize a variety of transaction types—from early stage investments to large-scale deals—given the competitive landscape, companies are going to need to revamp their business development process to drive more outcomes from their transactions.
So where are there opportunities for enhancement?
In this dynamic and unstable business climate, having a clear yet agile approach to evaluating all of your options will be key. Companies should continue to push harder on asking themselves whether products and services need to be made in-house. Focusing on differentiated capabilities that will really be the critical value drivers for the business can provide the strategic path towards helping to clarify what is often muddied.
Brand perception in the market will become more important as the business world moves forward. Internally competing views can lead to challenges, and so aligning company culture with the external market perception is critical. A real partner of choice can bring value in a manner that will make the decision obvious.
Having a clear strategy on therapeutic areas and technologies at times can be the simpler part of the equation. Driving this systematically to the external markets in a holistic manner can be challenging. Due to lack of clarity on prioritization (internal versus external projects, or both) and conflicting agendas (investment thresholds, rewards and personal objectives), disconnects can occur. Teaming will be critical to make sure everyone is swimming in the same direction. Companies that will succeed in the long term will find a way to have the right combination of “not made here” and “made here.” Finding the balance of free-spirited innovators combined with disciplined investors will be the key to efficient returns.
Setting up the right structure, focus and operations is likely not enough. Ensuring that the ecosystem can be agile to adjust to where the markets are moving and to business needs will be critical. Annual refreshes to the plan will be key, as well as good communication throughout and the ability to navigate in a fluid manner when necessary. Ensuring the partnership enablers that are market-facing are aligned and working together in an efficient manner with the organization can drive the best outcomes.
While creating capabilities to enhance competitive advantage and value for your business is critical, you must also ensure the business is protected from factors that can destroy value, such as cyberattacks, quality and compliance problems, legal matters and other ethical challenges. Driving technology-enabled digital change can be the most efficient path forward. Companies that have appropriately focused on the people and process side of the equation and are now addressing technology will be better equipped to manage threats.
Cybercrime continues to be a major focus of pharmaceutical company boards and executives as cyberattacks increase in sophistication. PwC’s 2022 Global Economic Crime and Fraud Survey (GECS) found that, among the 19 types of economic crime, cybercrime stood out as the most widespread—and most disruptive—event experienced over the past two years globally and domestically. Hackers are becoming increasingly sophisticated and are targeting large pharmaceutical companies, putting clinical discovery trial data, patient health information and other pharmaceutical trade secrets in jeopardy. For example, a 2020 cyberattack on the European Medicines Agency exposed data for a leading coronavirus vaccine.
We assess that the overall cyber risk to the pharmaceutical industry remains high due to the continued threat of ransomware. There has been a significant increase in ransomware attacks along with the solidification of ransomware-as-a-service groups as a preeminent threat. There is also a concurrent escalation of geopolitical tensions with a nation-state actor currently at odds with the US, which could significantly disrupt supply chain operations. Overall, nation-state actors continue to be a formidable threat, with many demonstrating interest in pharmaceutical development.
Given the broader labor changes, supply shortages and constantly changing supply chain strategies and operations, the focus on quality can be challenging to sustain, and the downside can have massive impacts on the business, including the potential inability to manufacture products.
Having high standards of ethics is not enough anymore. Given the challenging labor market and the need to drive better ESG results, ensuring that a company's core values and purpose are front and center and that employees feel the meaning and purpose of their work will be critical. Being bold and bringing back a feeling of pride to the industry will be critical not only to retaining current employees, but to becoming the employer of choice.
The winners in the industry will be able to optimize the human-technology interface by leveraging data across the enterprise to derive insights. For example, by using analytics to automate the batch release process, not just to mitigate risks, but to improve the manufacturing process and yields. Some companies are leveraging quality to improve clinical outcomes and accelerate the completion of clinical trials.
“Having high standards of ethics is not enough anymore.”
Given the global nature of the industry and the impact of tax on many aspects of the business, we see taxes continuing to be an area of value retention and creation. With potentially changing supply chains from geopolitical instability, tax structuring and planning will be critical to prevent leakage.
Although pharmaceutical companies got a reprieve in the US this March with changes to the corporate tax regime, global tax reform is still on the near horizon. Countries want their fair share of tax contributions from pharma companies and are applying pressure for more transparency and increasing the number of audits from government agencies. A two-pillar tax plan at the Organisation for Economic Co-operation and Development (OECD) is complete and is set to take effect in 2023. Maintaining a clear and defendable tax strategy that is agile given the environment will be key to long-term success. The winners will have tech-enabled, real-time scenario planning on tax built into broader investment decisions to ensure operational decisions have tax efficiencies embedded in the outcomes.
The pharmaceutical industry mission is more important than ever—vaccines are helping the world climb out of the COVID-19 pandemic, innovative medicines are healing our loved ones and clinical trials are providing hope for so many.
It is a privilege to serve this mission. Demands to do it well are rising, with discerning investors looking to back the winners. In this environment, executives should be asking themselves:
If you answered no to any of these questions, the time to act is now. Executive teams should ensure they have a game plan for transformation while also protecting the enterprise, in order to create the value patients and shareholders expect.
{{item.text}}

{{item.text}}